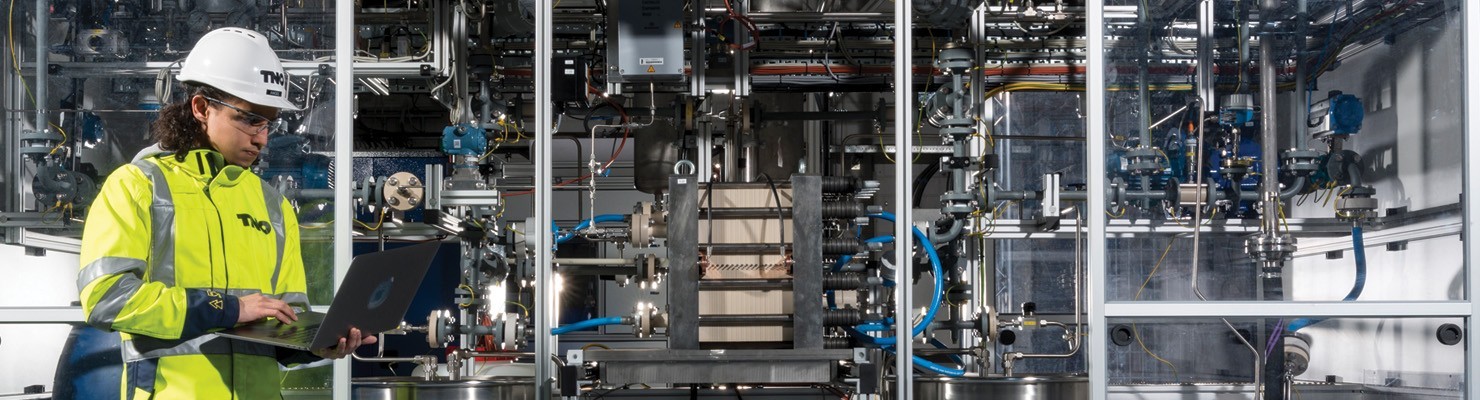
Driving on CO2: VoltaChem scales up sustainable production of formic acid
Carbon dioxide (CO2) is best known for its contribution to global warming. Less known is that it can also function as a resource for the production of renewable fuels and chemicals. For the next two years six partners in a consortium of Shared Innovation Program VoltaChem will collaborate in a project that aims to convert CO2 with the aid of sustainably produced electricity into large amounts of formic acid. This can be used as a fuel, as conserving agent in animal feed, to store energy and as a chemical building block. The result: lower production costs, lower CO2 emissions and a contribution to the sustainable energy system of the future.
Recycling of CO2 as a resource for the sustainable production of formic acid
Up until now formic acid is almost exclusively produced on the basis of fossil fuels. Previously it was demonstrated on a laboratory scale that is possible to produce formic acid with the aid of CO2 and electricity: direct electrochemical conversion of CO2. In this process water is converted into hydrogen and oxygen with the aid of electricity. Next, the hydrogen is directly used to convert the CO2 with the aid of electricity into formic acid.
This new project takes the next step towards the development of an industrial process. By the end of 2017 an electrochemical reactor will be delivered that can continuously produce formic acid on a small scale using fluctuating amounts of renewable energy (solar and wind). By 2018, on the basis of these results, VoltaChem wants to demonstrate that this process can also be deployed on an industrial scale.
Formic acid: carbon neutral and safe storage medium for fuels and energy
By using CO2 as a resource and by using sustainably produced energy you get carbon neutral formic acid. Another advantage is that you can use formic acid as a liquid and relatively safe storage medium for hydrogen, for instance as a fuel in vehicles. As it happens, hydrogen gas has the disadvantage that you have to store and transport it under high pressure and that it is highly flammable. To conclude you can use formic acid to store sustainably produced energy when there is an overproduction of wind- or solar energy.
Under the flag of Shared Innovation Program VoltaChem
This project is being executed under the flag of Shared Innovation Program VoltaChem: an initiative of the Dutch Top Sector Chemistry where companies and research institutes jointly work on the electrification of the chemical industry. Fossil energy and -resources are being replaced by renewable ones such as wind, solar and biomass for the production of chemical resources. The aim is to make the chemical industry more competitive, more innovative and more sustainable.
In the project 'Power-2-FA: Direct electrochemical conversion of CO2 to formic acid' for the next two years the following six organizations will collaborate:
- TNO is project coordinator on behalf of VoltaChem and will investigate how formic acid on the basis of CO2 with the aid of sustainably produced electricity can be cost effectively produced in a continuous industrial process on a large scale. TNO will also look at the influence of a fluctuating electricity supply on the efficiency of the system.
- Coval Energy has developed an innovative electrochemical technology to produce formic acid on the basis of CO2 and, together with TU Delft, they will demonstrate on a small scale that the technology is suitable to produce formic acid with the required quality and speed. Subsequently they will look at industrial scale-up.
- TU Delft will perform tests with the small-scale setup and will deploy fundamental expertise and advanced calculation models in the field of electrochemical reactions, materials and scaling-up in order to optimize the production of formic acid.
- CE Delft will investigate the entire value chain, from formic acid production to applications, including a life cycle analysis (LCA). They examine what it takes to close the carbon cycle and what will be the potential impact of the production of formic acid on the energy system of The Netherlands.
- Mestverwerking Friesland wants to convert the CO2 from its manure processing- and biomass installations into the valuable product formic acid. This project investigates how gas form the biogas installations of Mestverwerking Friesland can be used as a source of CO2.
- Team FAST is a TU/e student team that aims to drive a city bus on formic acid. The first 1-meter scale model has been realised and in July they will present the world's first city bus that drives on formic acid. For them it is vital to be able to sustainably produce the formic acid.
The entire chain, from CO2 as a resource to applications, and from fundamental to applied research is covered by this unique project. The aim of VoltaChem is to use the knowledge that will be obtained by this project broader for the purpose of industrial electrification to lower the emissions of greenhouse gases in the future and at the same time contribute to a sustainable energy system.
The project is co-financed by the Dutch Rijksdienst voor Ondernemend Nederland (RVO).
Indicative GHG-balance for formic acid as a hydrogen carrier in transport
One of the first project outcomes is a study by CE Delft. Please find a summary of the results below or read the full report.
The chemical formic acid (CH2O2) is an elementary chemical that can serve as a ‘hydrogen carrier’ for fuel cell powered drive trains.
In order to assess the sustainability merits of the application of formic acid in transport, this brief report describes an indicative greenhouse gas (GHG) footprint analysis, focussing on the GHG-emissions of the production and application life cycles.
To elucidate the effects of applied production routes, CO2-sources and energy sources, the following greenhouse gas balances are described:
- Conventional chemical formic acid production route (from carbon monoxide and water); this is the reference for formic acid production;
- Electrochemical formic acid production route (from electricity, water and CO2), in two variants that reflect (more or less) the extremes with respect to the applied power source and source of CO2:
- Using largely fossil fuel based electricity (current Dutch power grid mix) and fossil CO2;
- Using renewable electricity and a biogenic CO2 source.
In all cases, the comparison is made for application of the formic acid as a transport fuel in public transport buses, where also a comparison with the diesel fuel chain is made.
The results of the GHG-balances are shown in Figure 1.
Figure 1 GHG-balances

The results show that application of formic acid as a hydrogen carrier in transport only results in reduction of transport GHG-emissions if renewable electricity and biogenic CO2 are used in formic acid production. If conventional chemical or electrochemical formic acid (produced from grid electricity and fossil CO2), is used, then the well to wheel emissions are significantly higher compared to the diesel reference case.
The current state of art electrochemical production will, when using grid based power, result in about 10% higher greenhouse gas emission per unit of formic acid compared with conventional production. 3 May 2017 3.J68 - Indicative GHG balance for formic acid as a hydrogen carrier in transport
It is expected, however, that there is ample room to improve the process, which would also improve the GHG performance and may result in savings of over 50% compared to conventional formic acid, the reference for the production. In this case, replacement of conventional formic acid production by electrochemical formic acid will yield emission reduction, even if fossil power is used. But application in transport, where diesel would be the reference, would still result in a GHG emission increase.
Status of results and next steps
This study is a preliminary analysis of the power to formic acid life cycle, a more elaborate study is undergoing. The established results are indicative because of present limited availability of data.
In the ongoing study, more application areas are studied, including application in ships, stationary applications, more sensitivities are assessed, the impacts on (decentralized) renewable power systems are detailed, and we will put the technology in perspective of alternative sustainability options.
The study is part of the ‘Power 2 Formic Acid’ Joint Industry Project, which aims to develop the process innovations to bring the electrochemical reactor closer to the market. The Power 2 Formic Acid project is conducted under the VoltaChem shared innovation program by a consortium of TNO, TU Delft, Coval Energy, Team Fast, CE Delft and Mestverwerking Friesland, supported financially by RVO (Topsector Energiesubsidie van het Ministerie van Economische Zaken).
