

Water + air + electricity = hydrogen peroxide
08-12-2016
 Decentralised production of hydrogen peroxide from water, air and electricity. The feasibility of this approach has been demonstrated within the VoltaChem Shared Innovation Programme. The improved concept of the electrochemical production of hydrogen peroxide is more environmentally friendly, and reduces hazards. Four questions for TNO’s Roel Bisselink about the technology, its relevance and the future.
Decentralised production of hydrogen peroxide from water, air and electricity. The feasibility of this approach has been demonstrated within the VoltaChem Shared Innovation Programme. The improved concept of the electrochemical production of hydrogen peroxide is more environmentally friendly, and reduces hazards. Four questions for TNO’s Roel Bisselink about the technology, its relevance and the future.
1. Why was this technology developed?
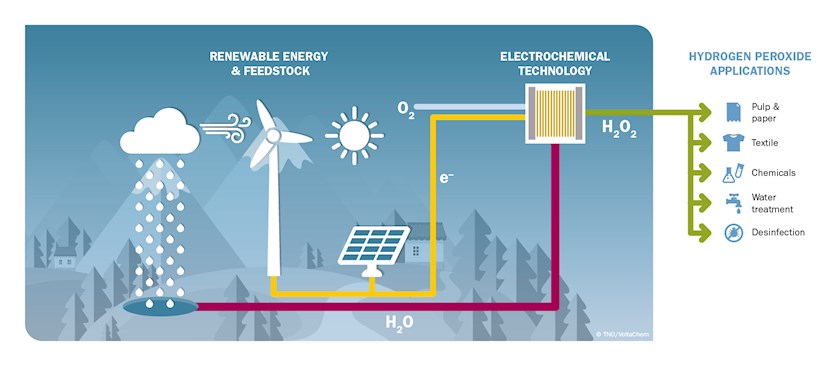
“Hydrogen peroxide has some particularly useful applications. These range from the bleaching of fibres in the paper, pulp and textile industries, to disinfection, the production of chemicals, soil remediation, and a wide range of water treatment processes. The substance breaks down into water and oxygen, and is more environmentally friendly than chlorine-based bleaches. However, the process traditionally used in the production of hydrogen peroxide consumes a great deal of energy, generates substantial wastes, and requires large volumes of organic solvents. Furthermore, there have been a string of incidents associated with the transport and handling of this substance. Decentralised, on-site production enables reduction of these hazards, provided that no use is made of organic solvents or hydrogen gas. For this reason, around fifteen years ago, TNO started development work on an electrochemical production method. An improved version of this technology was recently developed in the context of the VoltaChem programme.”
2. What is so special about this improved version?
“This is a genuine breakthrough. In the ‘conventional’ electrochemical method, oxygen, present in air, is reduced to hydrogen peroxide in an alkaline environment. We have now made it possible to produce hydrogen peroxide across a wide pH range, from acidic to neutral to basic. However, an electrolyte (a salt, acid or base) is required to conduct electricity. As a result, chemicals are consumed in this process and production takes place in a solution. With the decentralised approach, in which hydrogen peroxide is made on-site, co-dosing electrolyte is not desired. It could for example lead to increased salinity of a system, especially when considering closed systems. Our new concept involves less chemicals and virtually no electrolyte, thus increasing the sustainability of the process. The beauty of it is that the basic principle involved not only enables us to produce hydrogen peroxide more efficiently, it can also be used for the electrosynthesis of other chemical substances.”
3. What makes this development so relevant?
“As indicated, hydrogen peroxide plays an important part in many industrial and civil applications. The market is growing by more than 5% a year, prompted by the increased interest in sustainable solutions. The electrochemical process that has been developed is relatively simple, efficient and highly selective. This yields substantial savings in terms of production costs, energy consumption and waste production. In addition, the process is more cost effective on small scale and linearly scalable, which makes decentralised production possible. The decentralised approach minimizes any hazards, both during production and transport. Improved hydrogen peroxide quality is feasible due to absence of organic solvents and metal catalysts. Additionally, it is possible to utilise renewable, locally generated electricity. Thus, the electrochemical production of hydrogen peroxide from water and oxygen is a prime example of industrial electrification, which could inspire the electrification of other chemical processes.”
4. So what does the future hold in store?
“We have now demonstrated the principle on a laboratory scale. Using a small laboratory reactor, we were able to produce hydrogen peroxide continuously for a whole working week. Based on this result, we have confidence in the operation of the technology. Next year, we will be making further refinements to this reactor, after which we and our partners will scale up to an industrial reactor and an integrated demonstration system. Meanwhile, we are currently in discussion with several interested parties who wish to develop this technology further for specific applications. Furthermore, we have learned a great deal about the use of electrochemistry the design of electrochemical reactors capable of efficiently converting gases and liquids. We will certainly be putting this knowledge and experience to use in other processes. For instance, within the framework of the VoltaChem programme, we are already working on the conversion of CO2 to fuels and synthesis of the building blocks for plastics, an area that we will surely be hearing much more about in the upcoming years.”
VoltaChem
VoltaChem is a Shared Innovation Programme for the electrification of chemistry, initiated by TNO, the Energy research Centre of the Netherlands (ECN) and Topsector Chemistry. Its objective is to utilise renewable energy in the chemical industry for the production of heat, hydrogen and chemicals.
Want to join us?
Are you interested in the electrochemical production of hydrogen peroxide or similar products, can you see new uses for this technology, or would you like to be directly involved in the research? If so, please feel free to get in touch with us to arrange a detailed discussion or to join the VoltaChem community. We are interested in discussions with companies throughout the entire value chain, from chemical manufacturers and equipment manufacturers to end-users in various sectors. Our goal is to jointly explore new business opportunities, to aid developments and to find commercially viable niches in the market for technology in the field of electrification and electrosynthesis.
Source: TNO TIME
Share this page: