

Electrons-2-Chemicals - E2C
Publications
Front. Chem. Eng., 2, 22 December 2020, 22
The Techno-Economic Benefit of Sorption Enhancement: Evaluation of Sorption-Enhanced Dimethyl Ether Synthesis for CO2 Utilization
Galina Skorikova1, Marija Saric1, Soraya Nicole Sluijter1, Jasper van Kampen1, Carlos Sánchez-Martínez2 and Jurriaan Boon1*
1 Sustainable Technologies for Industrial Processes, Energy Transition, TNO, Petten, Netherlands
2 Sustainable Process & Energy Systems, Energy Transition, TNO, Delft, Netherlands
https://doi.org/10.3389/fceng.2020.594884
Abstract
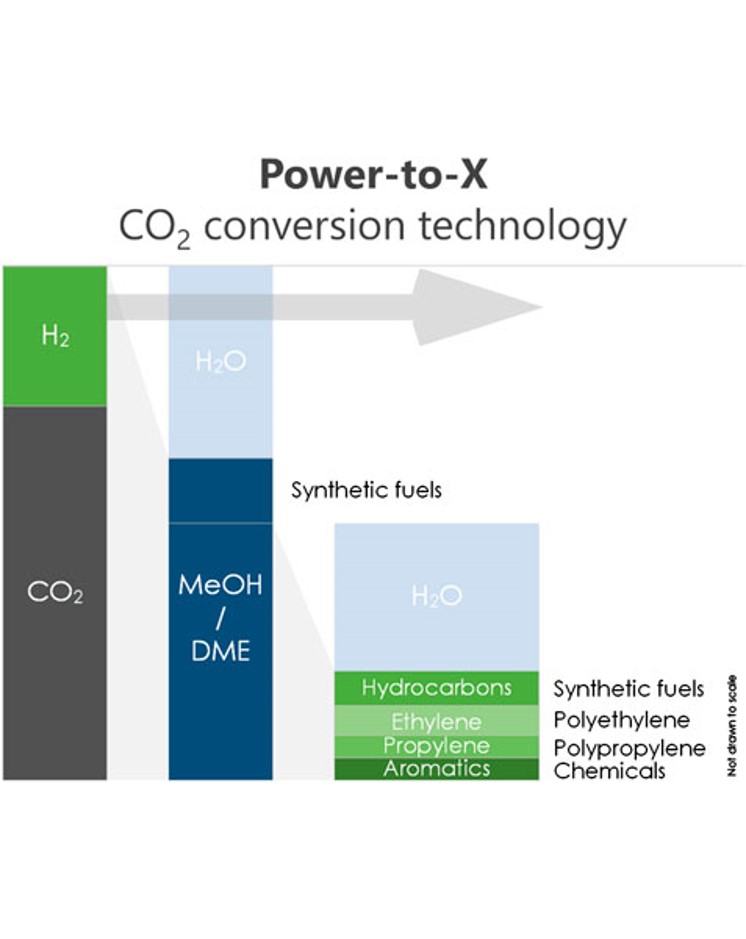
Dimethyl ether (DME) is an important platform chemical and fuel that can be synthesized from CO2 and H2 directly. In particular, sorption-enhanced DME synthesis (SEDMES) is a novel process that uses the in situ removal of H2O with an adsorbent to ensure high conversion efficiency in a single unit operation. The in situ removal of steam has been shown to enhance catalyst lifetime and boost process efficiency. In addition, the hydrogen may be supplied through water electrolysis using renewable energy, making it a promising example of the (indirect) power-to-X technology. Recently, major advances have been made in SEDMES, both experimentally and in terms of modeling and cycle design. The current work presents a techno-economic evaluation of SEDMES using H2 produced by a PEM electrolyzer. A conceptual process design has been made for the conversion of CO2 and green H2 to DME, including the purification section to meet ISO fuel standards. By means of a previously developed dynamic cycle model for the SEDMES reactors, a DME yield per pass of 72.4 % and a carbon selectivity of 84.7% were achieved for the studied process design after optimization of the recycle streams. The production costs for DME by the power-to-X technology SEDMES process at 23 kt/year scale are determined at ∼€1.3 per kg. These costs are higher than the current market price but lower than the cost of conventional DME synthesis from CO2. Factors with the highest impact on the business cases are the electricity and CO2 cost price as well as the CAPEX of the electrolyzer, which is considered an important component for technology development. Furthermore, as the H2 cost constitutes the largest part of the DME production cost, SEDMES is demonstrated to be a powerful technology for efficient conversion of green H2 into DME.

Energies, 13(24), 11 December 2020, 6556
Generic Dynamical Model of PEM Electrolyser under Intermittent Sources
Sumit Sood1, Om Prakash1, Mahdi Boukerdja1, Jean-Yves Dieulot1, Belkacem Ould-Bouamama1, Mathieu Bressel2 and Anne-Lise Gehin1
1 CRIStAL UMR CNRS 9189, Université de Lille, 59655 Villeneuve d’Ascq, France
2 CRIStAL UMR CNRS 9189, Junia, 59000 Lille, France
https://doi.org/10.3390/en13246556
Abstract
Proton Exchange Membrane (PEM) water electrolysis system is one of the promising technologies to produce green hydrogen from renewable energy sources (wind and solar). However, performance and dynamic analysis of PEM water electrolysis systems are challenging due to the intermittent nature of such sources and involved multi-physical behaviour of the components and subsystems. This study proposes a generic dynamical model of the PEM electrolysis system represented in a modular fashion using Bond Graph (BG) as a unified modelling approach. Causal and functional properties of the BG facilitate the formal PEM electrolyser model to adapt and to fit the different configurations of the electrolyser ranging from laboratory scale to industrial scale. The system-specific key parameter values are identified optimally for a laboratory-scale electrolyser system running on a multi-source energy platform using experimental data. The mean absolute percentage error between simulation and experimental data is found to be less than 5%. The performance characteristic curves of the electrolyser are predicted at different operating temperatures using the identified key parameters. The predicted performance is in good agreement with the expected behaviour of the electrolyser found in the literature. The model also estimates the different energy losses and the real-time efficiency of the system under dynamic inputs. With these capabilities, the developed model provides an economical mean for design, control, and diagnosis development of such systems.
Int. Journal of Hydrogen Energy, 45(46), 21 September 2020, 24232
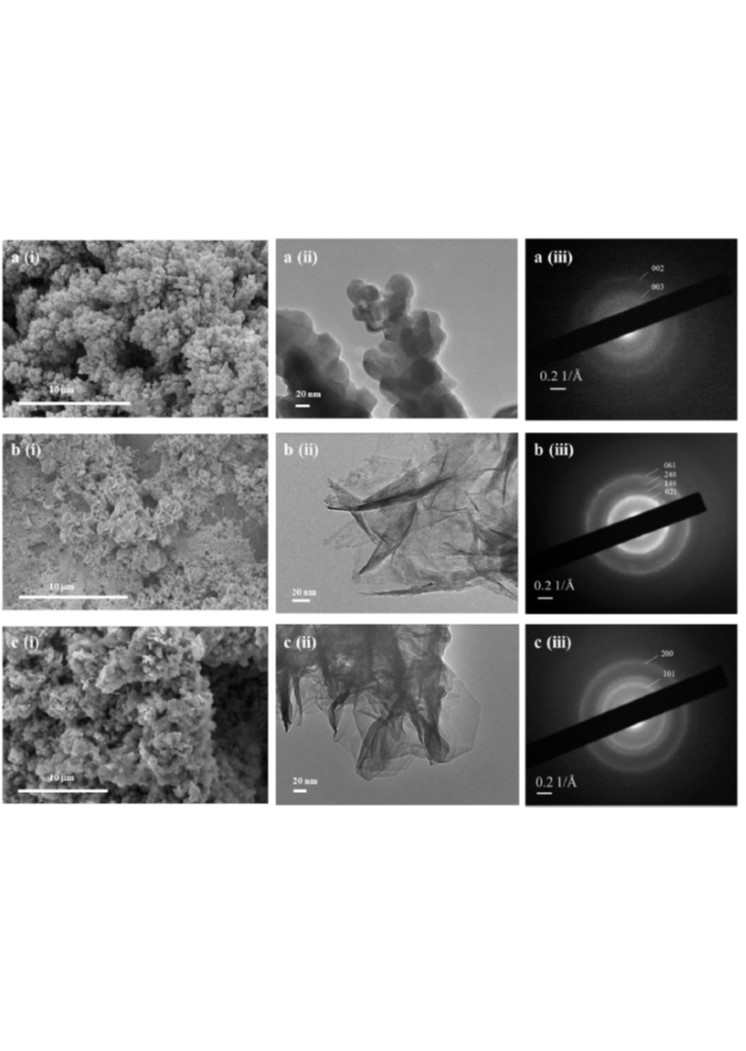
Development of Ni–Fe based ternary metal hydroxides as highly efficient oxygen evolution catalysts in AEM water electrolysis for hydrogen production
Adeline Loha, Xiaohong Lia, Oluwadamilola O. Taiwob, Farid Tariqb, Nigel P. Brandonb, Peican Wangc, Ke Xuc, Baoguo Wangc
a Renewable Energy Group, College of Engineering, Mathematics and Physical Sciences, University of Exeter, Penryn Campus, Cornwall, TR10 9FE, UK
b Earth Science and Engineering, Imperial College London, SW11 2AZ, UK
c Department of Chemical Engineering, Tsinghua University, Beijing, 100084, China
https://doi.org/10.1016/j.ijhydene.2020.06.253
Abstract
A number of mixed metal hydroxide oxygen evolution reaction (OER) catalysts i.e. Ni–Fe, Ni–Co, Ni–Cr, Ni–Mo, Ni–Fe–Co, Ni–Fe–Mo and Ni–Fe–Cr were prepared by cathodic electrodeposition and characterised by SEM, TEM, EDS, XPS and micro X-CT. The compositions of selected catalysts were optimised to give lower OER overpotentials in alkaline media. Further optimisation of Ni–Fe based ternary metal hydroxide catalysts such as Ni–Fe–Co and Ni–Fe–Mo was carried out, showing improved performance at high current densities up to 1 A cm−2 in 1 M NaOH, 333 K. The influence of electrodeposition parameters such as current density, pH, electrodeposition time and temperature on the electrocatalytic performance of ternary Ni–Fe–Co metal hydroxide was further investigated and optimised. The durability of the optimised catalyst was tested at a current density of 0.5 A cm−2 in an anion exchange membrane (AEM) water electrolyser cell at 4 M NaOH, 333 K, demonstrating stable performance over 3.5 h.
Journal of CO2 Utilization, 37, April 2020, 295-308
Sorption enhanced dimethyl ether synthesis for high efficiency carbon conversion: Modelling and cycle design
Jasper van Kampena,b, Jurriaan Boona,b, Jaap Ventea, Martin van Sint Annalandb
a Sustainable Process Technology, ECN part of TNO, P.O. Box 15, 1755ZG Petten, the Netherlands
b Chemical Process Intensification, TU/e, P.O. Box 513, 5600MB Eindhoven, the Netherlands
https://doi.org/10.1016/j.jcou.2019.12.021
Abstract
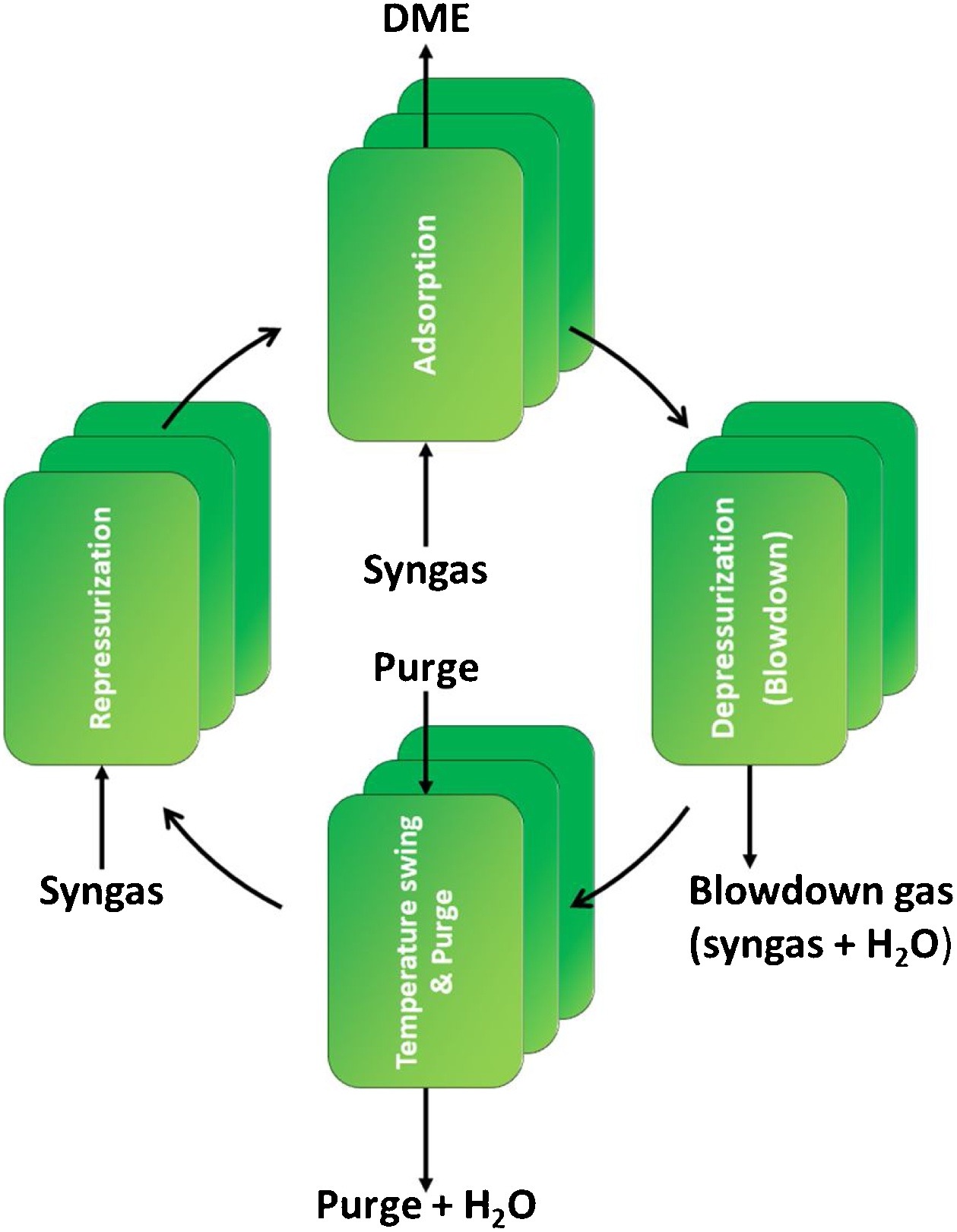
Dimethyl ether is one of the most promising alternative fuels under consideration worldwide. Both the conventional indirect DME synthesis and the improved direct DME synthesis process are constrained by thermodynamics, which results in limited product yield, extensive separations and large recycle streams. Sorption enhanced DME synthesis is a novel process for the production of DME. The in situ removal of H2O ensures that the oxygen surplus of the feed no longer ends up in CO2 as is the case for direct DME synthesis. As a result CO2 can be converted directly to DME with high carbon efficiency, rather than being the main byproduct of DME production.
The sorption enhanced DME synthesis process is a promising intensification, already achieving over 80 % single-pass CO2 conversion for a non-optimized system. The increased single-pass conversion requires less downstream separation and smaller recycle streams, especially for a CO2-rich feed. A key optimization parameter for the process performance is the adsorption capacity of the system. This capacity can be improved by optimizing the reactive adsorption conditions and the regeneration procedure. In this work, a detailed modelling study is performed to investigate the impact of various process parameters on the operating window and the interaction between different steps in a complete sorption enhanced DME synthesis cycle, and to compare its performance to other direct DME synthesis processes. The development of sorption enhanced DME synthesis, with its high efficiency carbon conversion, could play a significant role in the energy transition in which the carbon conversion will become leading.
Chemical Engineering Journal, 378, December 2019, 122224
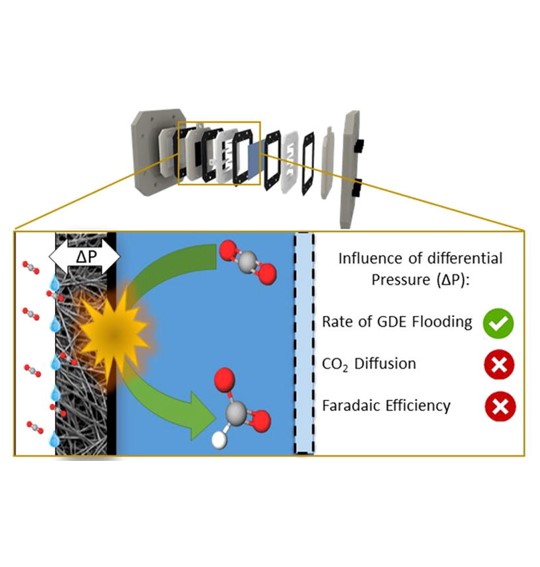
Influence of flow and pressure distribution inside a gas diffusion electrode on the performance of a flow-by CO2 electrolyzer
Bert De Mota, Jonas Hereijgersa, Miguel Duartea, Tom Breugelmansa,b
a Research Group Applied Electrochemistry & Catalysis, University of Antwerp, Universiteitsplein 1, 2610 Wilrijk, Belgium
b Separation & Conversion Technologies, VITO, Boeretang 200, 2400 Mol, Belgium
https://doi.org/10.1016/j.cej.2019.122224
Abstract
Over the last few years, the scientific community has paid lots of interest in the electrochemical CO2 reduction (ECR) as a possible solution for earth’s global warming and the transitioning to a CO2 neutral industry. The majority of the researchers focus on improving the catalyst material, leading to higher current densities at lower cell potentials and increased selectivities. However, so far, little attention has been given to the investigation and optimization of the ECR reactor design and process parameters, which are equally, if not more, important in order to up-scale the process towards an industrial level. In this work the ECR towards formate on tin nanoparticles in a flow-by electrolyzer is discussed. Special attention is given to the lay-out of both the reactor and the overall process. Additionally, the influence of the differential pressure across the gas diffusion electrode (GDE) on the reactor performance is investigated. It was found that by controlling the differential pressure, the perspiration, or leaking of electrolyte through the GDE, could be controlled and minimized. Results show that by controlling the differential pressure at 0 mbar, perspiration is minimal and that an optimal CO2 diffusion can be achieved, leading to an overall FE of 76% over 6 h at 100 mA/cm2. Furthermore, due to the electro-wetting effect of the GDE, it was impossible to operate the reactor without perspiration, thereby avoiding the risk of salt crystallization in the reactor. Overall this study strengthens the idea that flow-by electrolyzers are promising reactors for the industrialization of electrochemical CO2 conversion and that pressure regulation is essential to obtain optimal process conditions.
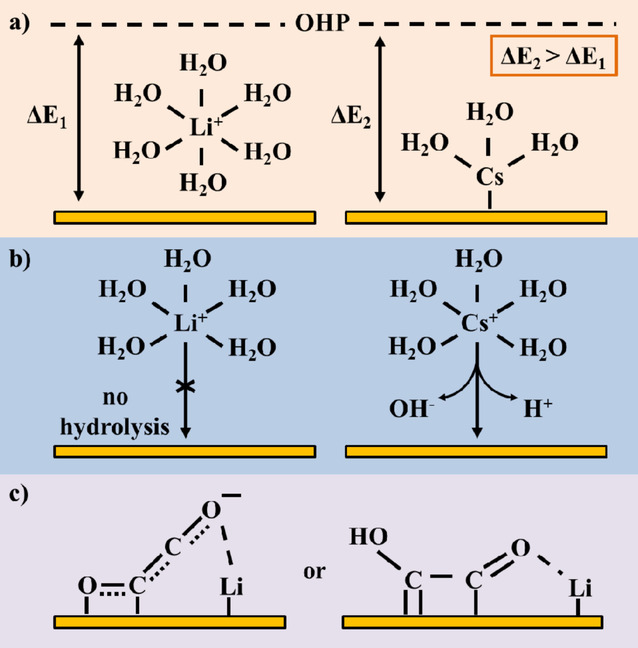
ChemPhysChem, 2019, 20, 2926
Electrolyte Effects on the Electrochemical Reduction of CO2
Marilia Moura de Salles Pupoa, Ruud Kortlevera
a Department of Process & Energy Faculty of Mechanical, Maritime & Materials Engineering, Delft University of Technology, Leeghwaterstraat 39, 2628 CB Delft, The, Netherlands
https://doi.org/10.1002/cphc.201900680
Abstract
The electrochemical reduction of CO2 to fuels or commodity chemicals is a reaction of high interest for closing the anthropogenic carbon cycle. The role of the electrolyte is of particular interest, as the interplay between the electrocatalytic surface and the electrolyte plays an important role in determining the outcome of the CO2 reduction reaction. Therefore, insights on electrolyte effects on the electrochemical reduction of CO2 are pivotal in designing electrochemical devices that are able to efficiently and selectively convert CO2 into valuable products. Here, we provide an overview of recently obtained insights on electrolyte effects and we discuss how these insights can be used as design parameters for the construction of new electrocatalytic systems.
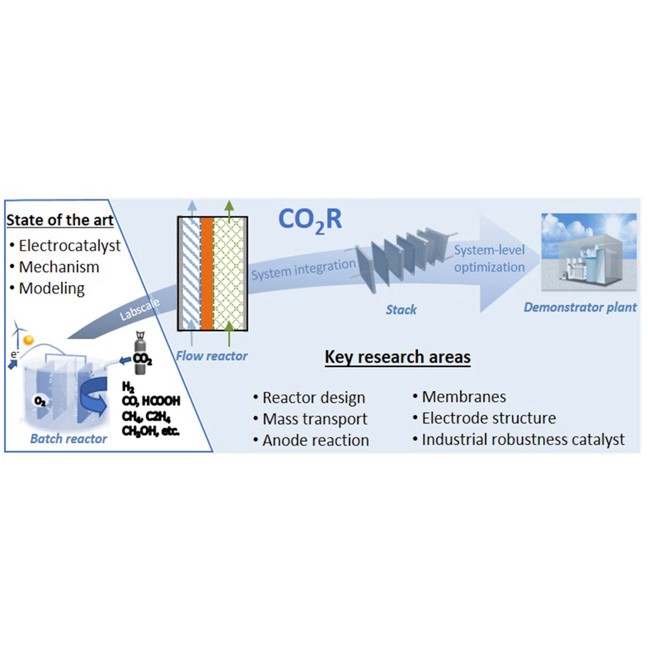
Current Opinion in Green and Sustainable Chemistry, 16, April 2019, 47-56
Recent advances in industrial CO2 electroreduction
Oriol Gutiérrez Sánchez1,2, Yuvraj Y. Birdja1, Metin Bulut1, Jan Vaes1, Tom Breugelmans1,2, Deepak Pant1
1 Separation and Conversion Technology, Flemish Institute for Technological Research (VITO), Boeretang 200, Mol 2400, Belgium
2 University of Antwerp, Research Group Advanced Reactor Technology, Universiteitsplein 1, 2610 Wilrijk, Belgium
https://doi.org/10.1016/j.cogsc.2019.01.005
Abstract
In light of the energy transition, electrochemical CO2 reduction (CO2R) has commonly been postulated as a favorable strategy for renewable energy storage and electrification of the chemical industry. However, for an effective impact of CO2R, large-scale implementation of this technology is required. The majority of research in this field has focused on fundamental and mechanistic understanding of the CO2R reaction and on the development of highly active and selective catalytic materials. Herein, we review the current status of the technology and discuss very recent developments and remaining challenges from an industrial perspective. We underscore the importance of system-level investigation and optimization of CO2R aimed at industrialization of the technology.