

An industrial perspective for the sustainable electrochemical production of tartaric acid: From CO2 to a C4 molecule
21-09-2020 | P2Chemicals | Research result
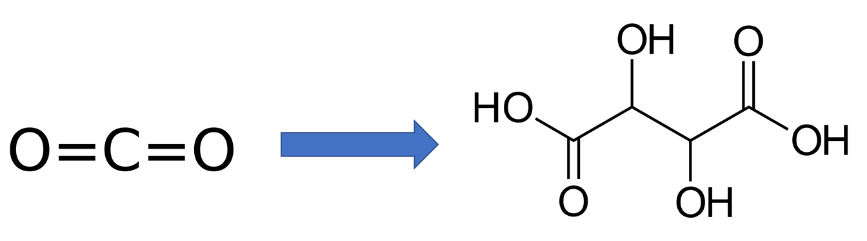 In a recently published paper in the ACS journal Sustainable Chemistry & Engineering, Amanda Garcia, Carlos Sánchez-Martínez, Ivan Bakker and Earl Goetheer present a feasible green and sustainable electrochemical production route for tartaric acid. The VoltaChem researchers synthesized this industrially relevant chemical through electrochemical reduction of oxalic and glyoxylic acids. In a techno-economic analysis they provide an outlook towards the electrochemical synthesis of tartaric acid on an industrial scale.
In a recently published paper in the ACS journal Sustainable Chemistry & Engineering, Amanda Garcia, Carlos Sánchez-Martínez, Ivan Bakker and Earl Goetheer present a feasible green and sustainable electrochemical production route for tartaric acid. The VoltaChem researchers synthesized this industrially relevant chemical through electrochemical reduction of oxalic and glyoxylic acids. In a techno-economic analysis they provide an outlook towards the electrochemical synthesis of tartaric acid on an industrial scale.
The research is a poignant example of the revival of electrochemical methods for the synthesis of organic molecules, enabling the direct use of electricity to generate valuable compounds. This is an important first step towards large-scale electrification of the chemical sector and ultimately the transition towards a sustainable society based on green, renewable processes rather than fossil-based ones.
Tartaric acid is an important product used in the textile printing, dyeing, pharmaceutical, and food industries, representing a current market value of around 220 million US dollar. It is a natural product found in the juice of grapes and as such a by-product in the wine industry. In the chemical industry, tartaric acid is produced by oxidation of carbohydrates and maleic acid. In their paper, that was recently published in SusChemEng, the VoltaChem researchers now present an alternative, sustainable electrosynthesis route based on the use of CO2. This can be electrochemically reduced to oxalic acid, glyoxylic acid and ultimately tartaric acid. This basically provides a route from a C1 molecule (CO2) to a C4 molecule. Upon transforming CO2 to tartaric acid, mass is for a significant part conserved.
Reaction pathway
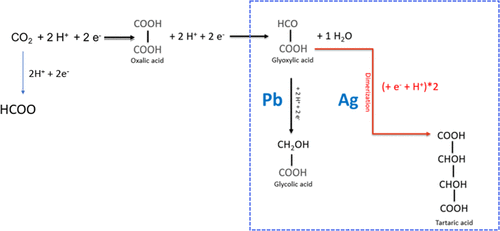 Although the electrochemical synthesis of chemical building blocks from CO2 has gained a lot of attention, surprisingly little is known about the reaction pathway towards tartaric acid. The VoltaChem researchers are the first to elucidate important details of this pathway. The SusChemEng paper describes the electrosynthesis starting from oxalic acid and glyoxylic acid, both in aqueous and acetonitrile solvents, and using silver as well as lead electrodes. Oxalic acid can relatively easily be made from CO2 with high efficiency. The best results were obtained using silver based electrodes, both for oxalic acid and glyoxylic acid as starting material. Here, the reduction reaction is more favourable toward the dimerization step, leading to tartaric acid. On the lead electrode, production of glycolate is favoured.
Although the electrochemical synthesis of chemical building blocks from CO2 has gained a lot of attention, surprisingly little is known about the reaction pathway towards tartaric acid. The VoltaChem researchers are the first to elucidate important details of this pathway. The SusChemEng paper describes the electrosynthesis starting from oxalic acid and glyoxylic acid, both in aqueous and acetonitrile solvents, and using silver as well as lead electrodes. Oxalic acid can relatively easily be made from CO2 with high efficiency. The best results were obtained using silver based electrodes, both for oxalic acid and glyoxylic acid as starting material. Here, the reduction reaction is more favourable toward the dimerization step, leading to tartaric acid. On the lead electrode, production of glycolate is favoured.
Techno economic analysis
In order to evaluate the commercial feasibility of an industrially relevant process for the production of tartaric acid using the reaction pathway described above, the researchers performed a techno-economic analysis (TEA). Based on best case scenario values for Faradaic efficiency, product yield, and current density, they conclude that there's indeed industrial potential for the electrosynthesis of tartaric acid.
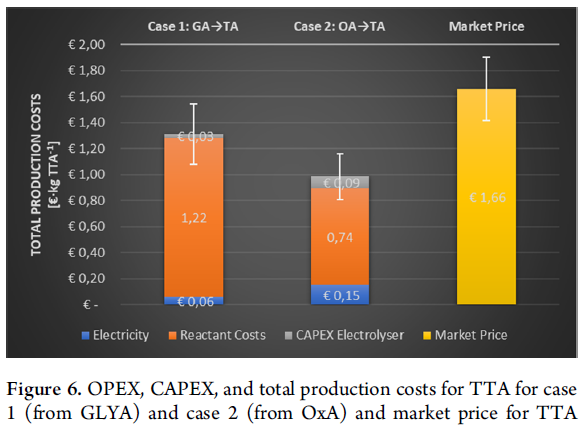 The most remarkable cost is the reactant cost, representing 93 and 75% of the total production costs for production from oxalic acid and glyoxylic acid, respectively. In both cases, the process economics are dominated by the price of the starting materials, indicating that the selected process conditions to run the reaction minimize the CAPEX and electricity consumption. It is evident that for a fully integrated process, i.e. starting from CO2, the reactant cost will be significantly reduced. This will lead to a good potential for a business case.
The most remarkable cost is the reactant cost, representing 93 and 75% of the total production costs for production from oxalic acid and glyoxylic acid, respectively. In both cases, the process economics are dominated by the price of the starting materials, indicating that the selected process conditions to run the reaction minimize the CAPEX and electricity consumption. It is evident that for a fully integrated process, i.e. starting from CO2, the reactant cost will be significantly reduced. This will lead to a good potential for a business case.
It has to be noted that the analysis does not include a downstream processing strategy for purification of the product. The performed TEA is only intended to give a preliminary order of magnitude estimation of the production costs.
Paper
Amanda C. Garcia, Carlos Sánchez-Martínez, Ivan Bakker, and Earl Goetheer: Sustainable Electrochemical Production of Tartaric Acid. ACS Sustainable Chem. Eng. 2020, 8, 28, 10454–10460 DOI: 10.1021/acssuschemeng.0c02493
Would you like more information on our efforts in Power-2-Chemicals, please contact our business developers Ties van Maaren or Reiner Grimbergen. For more information on the study presented here, please contact Earl Goetheer, technical lead Power-2-Chemicals.
Share this page:
Contact:

Reinier Grimbergen
Principal Consultant Power-2-X
+31 6 271 438 18
reinier.grimbergen@voltachem.com
LinkedIn