

e-Refinery symposium; towards CO2 neutral chemicals and energy
11-01-2018 | Event report | P2Fuels | P2Chemicals

e-Refinery
There is no silver bullet! Many roads have to be followed for the energy transition: there is not a single, simple solution.” According to one the speakers at the e-Refinery symposium on 18 December at Delft University of Technology.
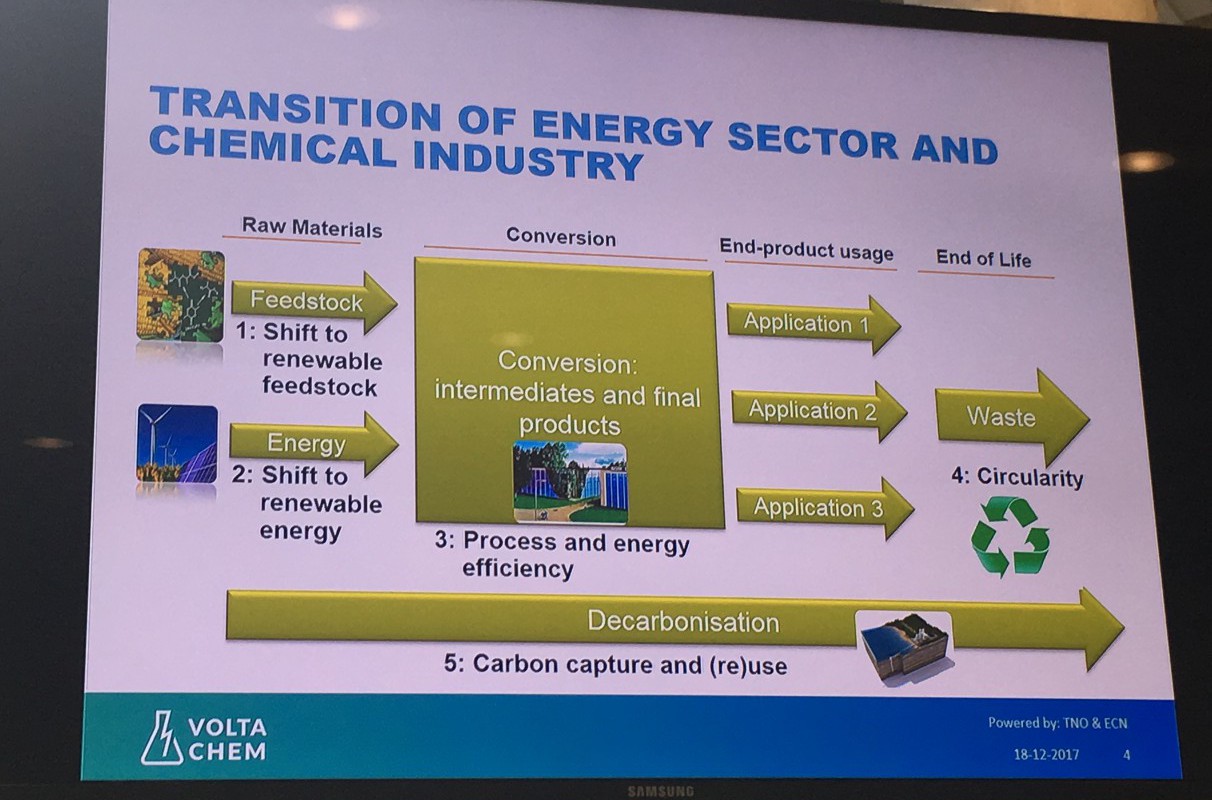
There is, however, with e-Refinery an overarching vision on the future of sustainable chemicals and fuels; the transition of the chemical and energy sectors from fossil fuels to renewable feedstock and electricity, the latter preferably from renewable sources such as wind and solar power. This will form the basis of an extensive network of chemical and energy conversions; although it’s not yet exactly clear what it will look like in detail.
Under the chairmanship of Delft chemist Bernard Dam, approximately one hundred seventy researchers, representatives of companies, and other interested parties were present in Delft to discuss the scientific challenges of this development.
Technology Readiness Level (TRL)
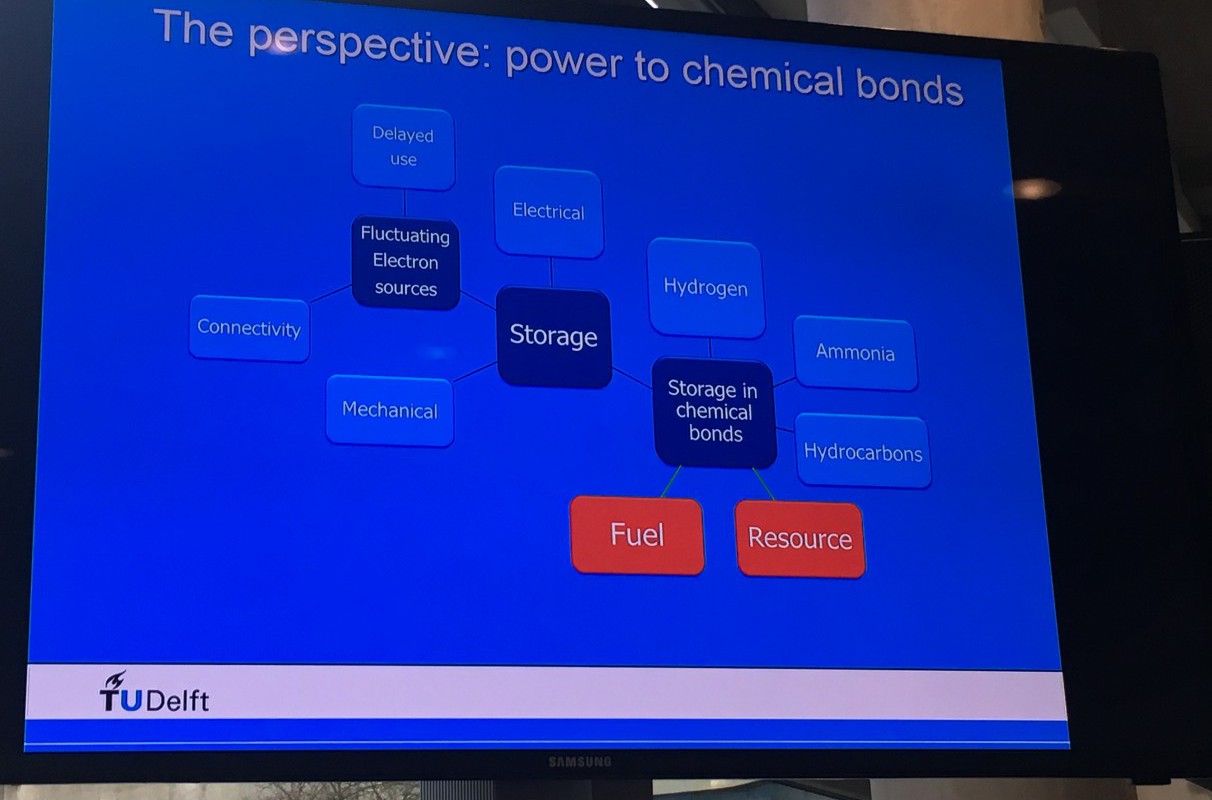
Scientists have already shown that the individual steps of an e-Refinery are possible. By using electrolysis you can turn water into hydrogen on an industrial scale: one electrode produces oxygen and the other hydrogen, useable as fuel or as a chemical feedstock.
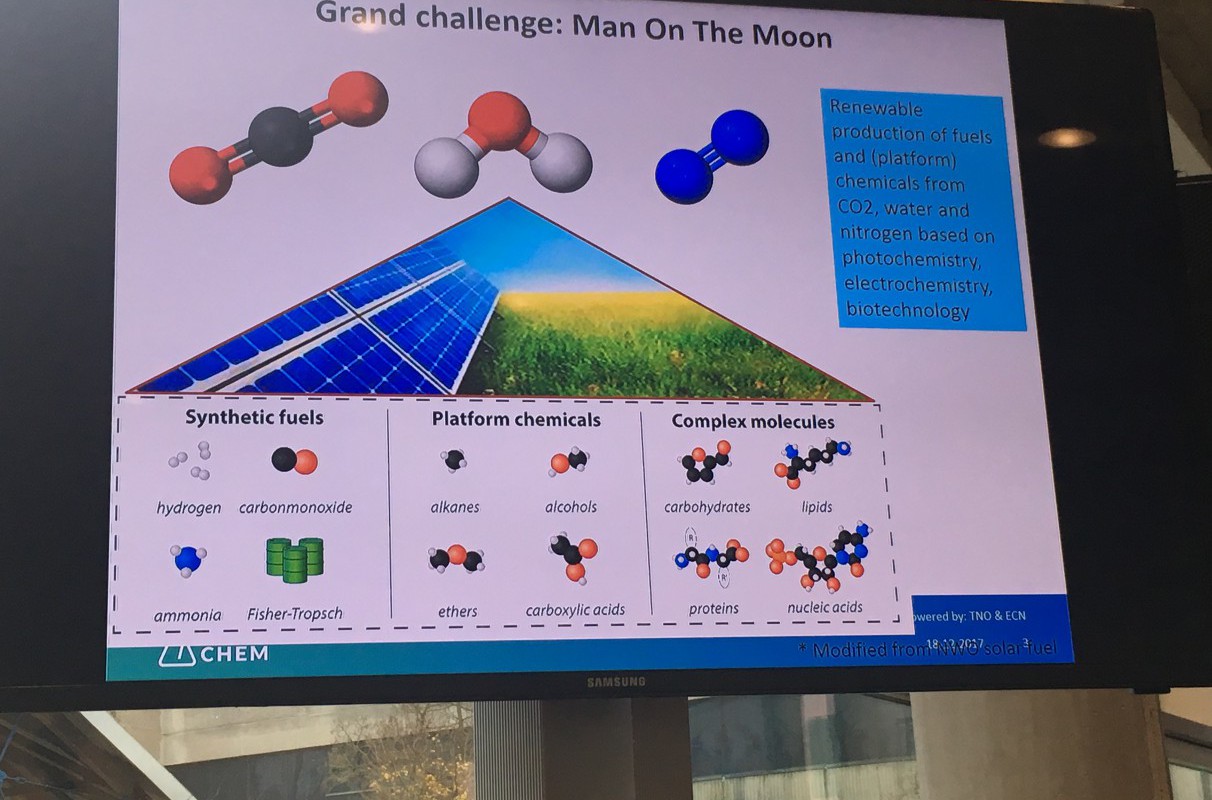
But further steps are needed, such as the conversion of carbon dioxide into fuels and other organic chemical feedstock. Or, for example, coupling (linking?) hydrogen with nitrogen to form ammonia. Many of these processes are technically possible, but the Technology Readiness Levels are still too low for industrial application and there are still fundamental research questions. So how do we accelerate these technologies to practice?
Steps are being made in this direction, revealed speaker Geert Laagland, by big Swedish energy company Vattenfall, which plans to produce ammonia, on a large scale, electrochemically. Shell is also making moves, having set up its own electrochemical laboratory in Amsterdam; the company’s Joep Huismans gave an overview of how Shell is engaging with the development of an e-Refinery concept. “This is a marathon,” he said, “not a sprint.”
Doggersbank
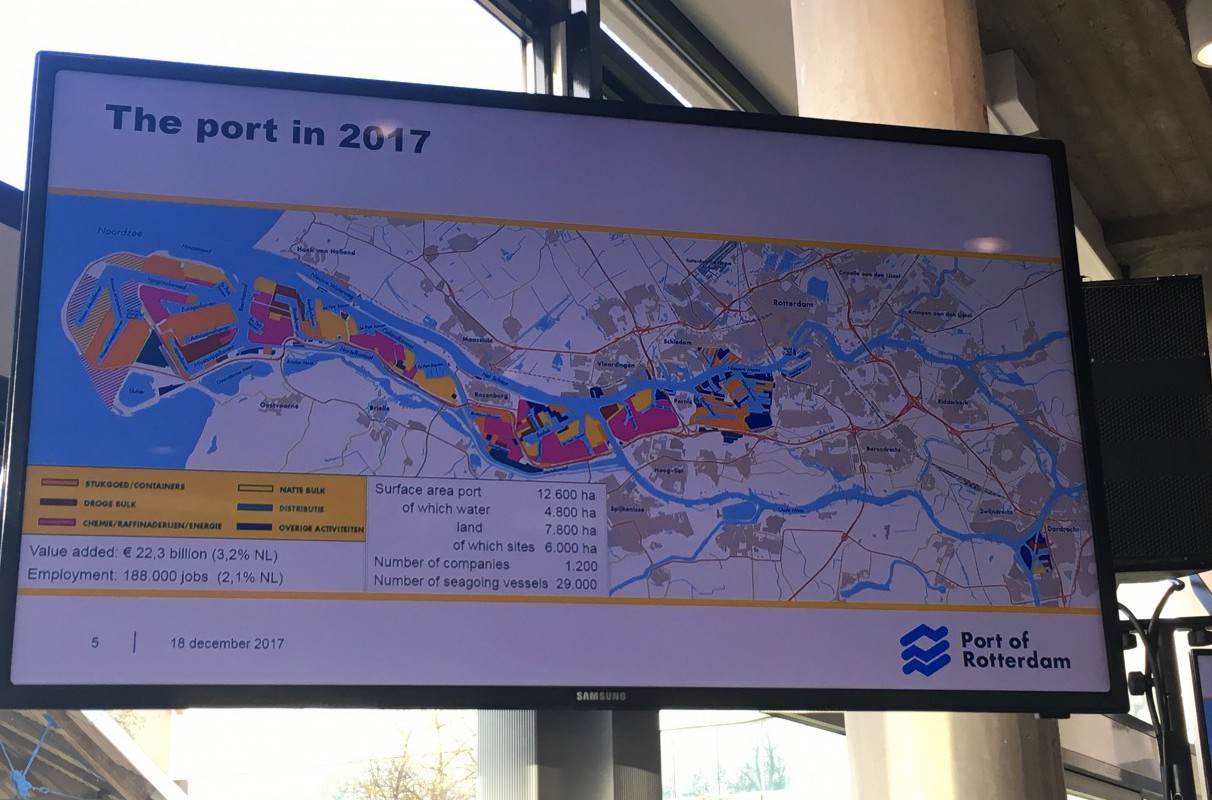
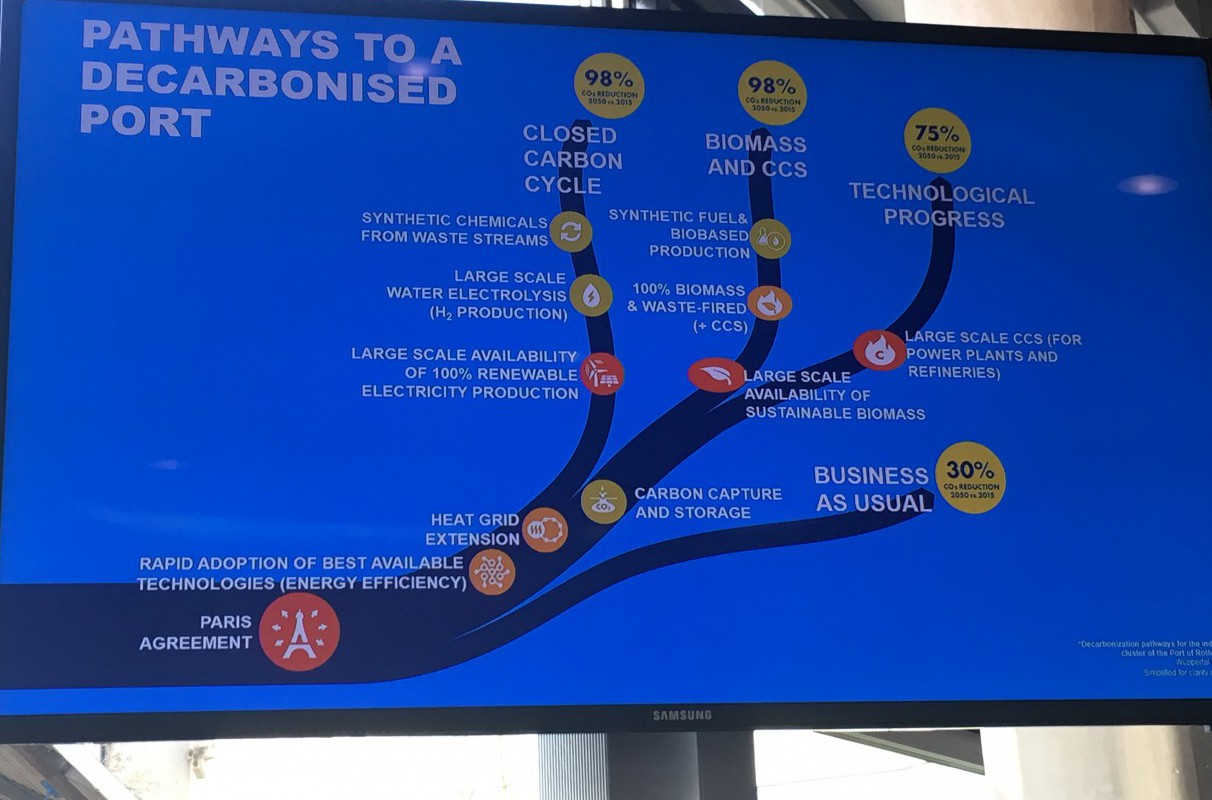
Ruud Melieste of the Port of Rotterdam shared his vision for the city’s port area. The chemical and energy industry will be 100% renewable in 2032, he predicted, using the Fischer-Tropsch chemical process to produce fuels from carbon dioxide, water, and electricity. The city will be heated by waste heat from industry. Visitors will be able to make excursions from Rotterdam to the wind energy hub at Dogger Bank in the North Sea, where a substantial proportion of the country’s energy will be produced. Carbon dioxide will be stored in empty gas fields under the North Sea; a necessary measure according to a number of speakers.
Wilson Smith of Delft Technical University presented detailed electrochemical research, on catalyst materials and membranes for electrochemical cells, and also by Guido Mul of Twente University and Earl Goetheer of the TNO/VoltaChem research institutes.
Techno-economic analysis
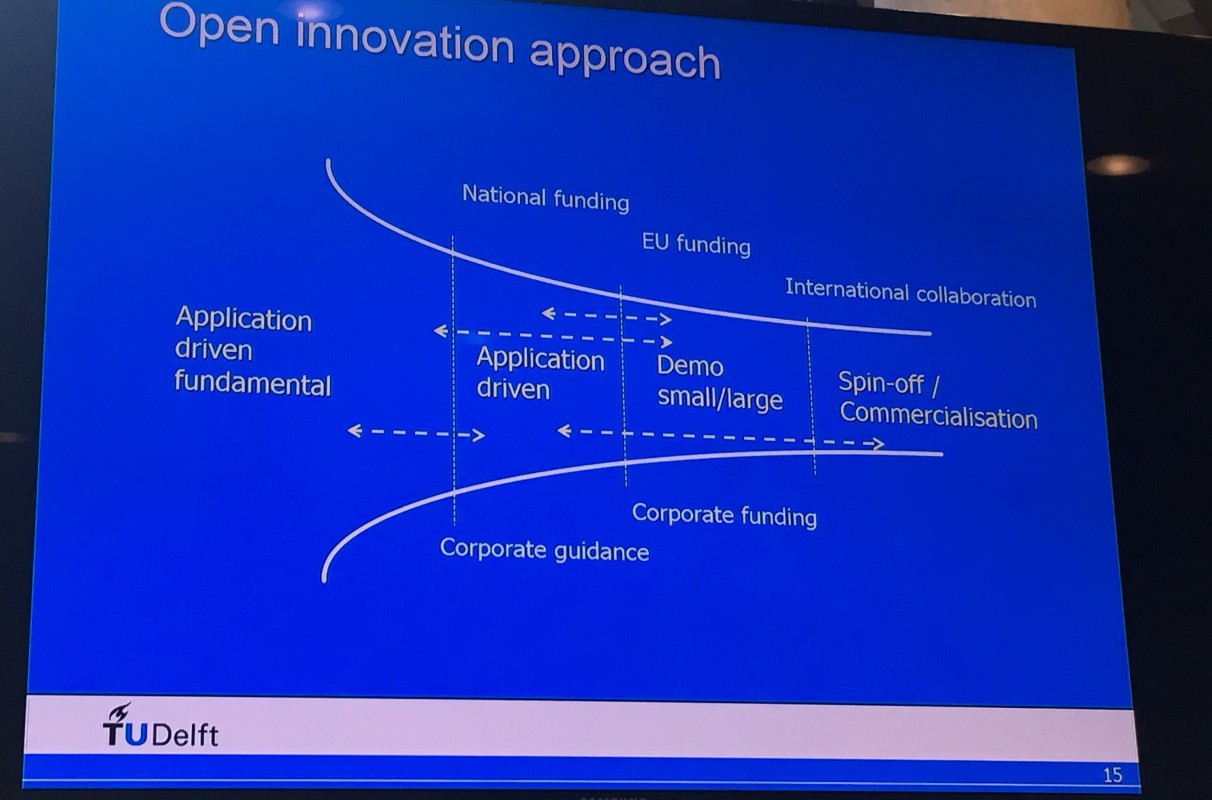
Matthias Wessling of RWTH Aachen gave an overview of new electrode materials and designs, such as those that promote mixing within electrochemical cells. Paul Kenis of the University of Illinois addressed the energy efficiency of various electrochemical cells, before turning to a techno-economic analysis, gauging the economic viability of scaling up processes from lab to industry.
Andrea Ramirez of TU Delft provided a full systems approach, advocating the integration of research at several levels: from electrochemical reactions and reactors to the regulatory framework, economic concerns and social issues such as society’s acceptance of new technologies.
Unknown unknowns
“Scaling up a chemical process from the laboratory to business application is no small and far from a trivial challenge,” said Ramirez. Unexpected complications have to be taken into account: the infamous ‘unknown unknowns of American defence secretary Donald Rumsfeld. “Nevertheless, even these can be anticipated by making comparisons with reference scaling processes, the results of which we already know.”, argued Ramirez.
At the same time, it’s necessary to take large-scale considerations into account when determining details. “Solar and wind energy are intermittent: you get peaks and sometimes you get nothing at all. This is well suited to batch processes, where limited quantities of a particular product are produced, whereas the chemical industry generally prefers continuous production processes.”
The final panel debate addressed propositions such as “eventually, all CO2 will have to be captured from the air” (probably); “the future is electric” (probably not) and “CO2 must be priced realistically”. Most panel members found this last proposition necessary for the development of a carbon-free chemical sector.
Bringing worlds together
“Personally, I found many of the talks were very scientific and detailed, but the general overviews were of great value”, said Rob Stevens, VP Technology Scouting with the ammonia producer Yara International. “But it's good that these people are brought together, and that a vision is growing that we have to electrify and decarbonize.”
'It's great”, says Martijn de Graaff, business development manager at co-organizer VoltaChem, “to see that the scientific world is starting to align more and more and that exchanges are beginning to emerge with the business community. This will support one of the primary goals of our Shared Innovation Program ‘VoltaChem’. To further develop the academic results of fundamental research and bring these results - at a relevant scale - into industrial practice. Preferably as quickly as possible, because time is not on our side.”
Another observation was that all different levels and perspectives were in the room. “There was great variety”, said Paulien Herder, chair of the Delft Energy Initiative of Delft Technical University. “For example the story of Andrea Ramirez. I enjoyed her contribution, which demonstrated how a system level could influence the chemical details. That’s a good fit for the audience, too”, she continued. “To bring this community together at all these system levels, and to keep them interacting, that’s exactly what this symposium was for.”
For a Dutch report and to see a special 'explanimation' video about e-refinery look at this TU-D page







Share this page: