

Integrated capture and conversion of CO2
04-11-2019 | New Project | P2Chemicals
A viable approach to efficient recarbonization
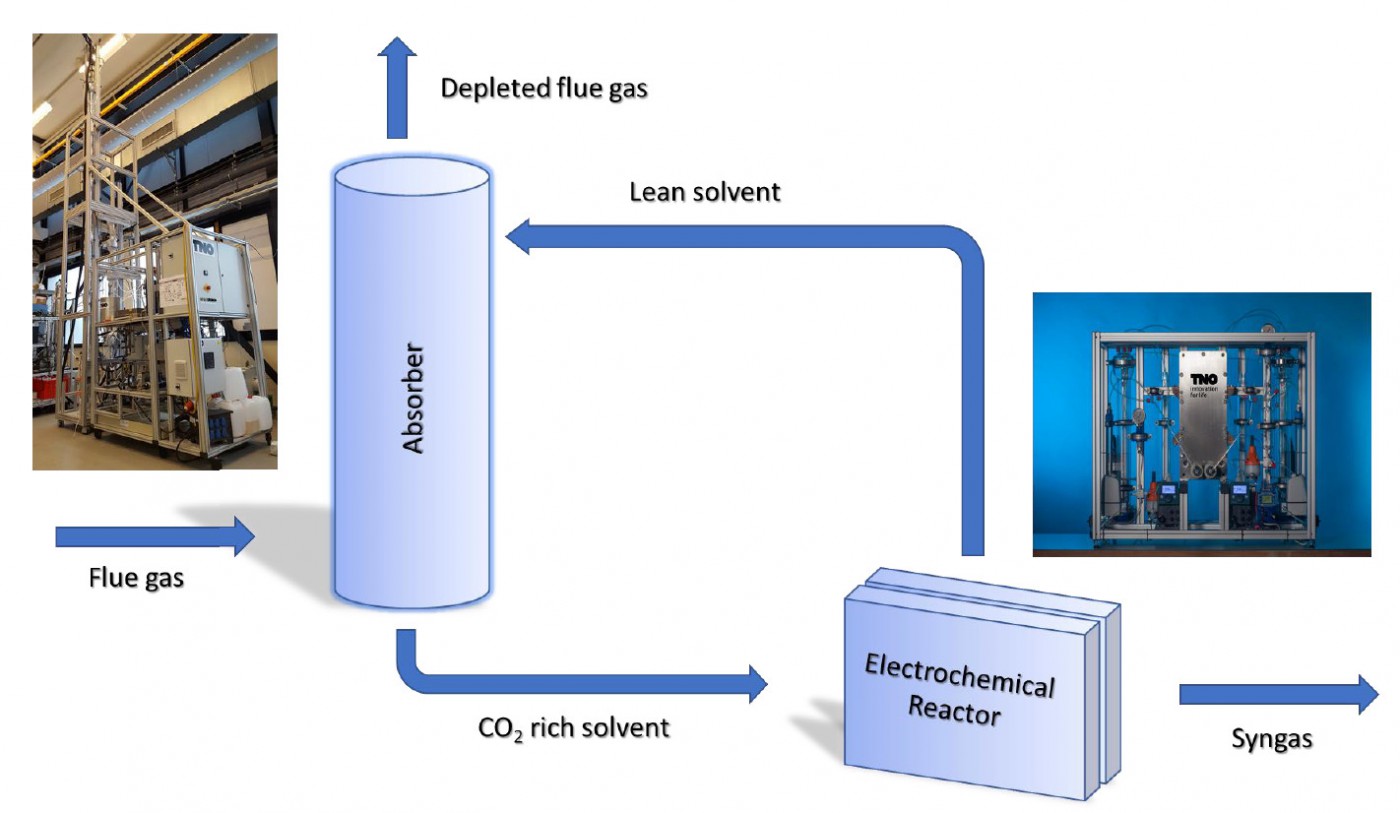 The direct use of CO2 loaded amine-based capture solvents in an electrochemical reactor is a viable concept for providing the chemical industry with sustainable, circular carbon-based starting materials. Laboratory experiments in VoltaChem's program line Power-2-Chemicals have already established high efficiencies in the capture integrated conversion of CO2 into formic acid. Now, a shared research program is being prepared to accelerate the development of this integrated approach.
The direct use of CO2 loaded amine-based capture solvents in an electrochemical reactor is a viable concept for providing the chemical industry with sustainable, circular carbon-based starting materials. Laboratory experiments in VoltaChem's program line Power-2-Chemicals have already established high efficiencies in the capture integrated conversion of CO2 into formic acid. Now, a shared research program is being prepared to accelerate the development of this integrated approach.
Using CO2 as a raw material renders chemical production more sustainable. Moreover, it helps to close the carbon cycle, thus contributing to a circular economy. Technology for this concept of recarbonization is being developed in VoltaChem's program line Power-2-Chemicals. But there is still an important challenge in creating a viable value chain in converting captured CO2 to chemicals.
Now, a new concept is being developed at Voltachem that can significantly improve cost-effectiveness through the direct use of amine CO2 capture solvents in an electrochemical reactor, thus offering an integration of the two processes of capture and conversion of CO2. According to VoltaChem's technical lead Power-2-Chemicals Prof. Earl Goetheer, the main gain of the novel approach is that a separate CO2 stripping unit, which adds to equipment costs and which consumes lots of energy, can be avoided. "If we look at the cost levels, currently captured CO2 simply is too expensive to be used as a commodity for the chemical industry. We propose in fact a process intensification that will significantly improve the economy of the overall process. Moreover, the technology matches the chemical industry not only in terms of cost but also in terms of scale."
Chemical equilibria at hand
The idea behind the integration is to use the elevated temperatures of an electrolyzer to partly recover CO2 from the amine-based capture solvent. "In CO2 scrubbers, recovery is most effective at temperatures of around 120 °C", Goetheer says. "But since we have chemical equilibria at hand, there already is substantial CO2 desorption at the temperatures of 60-80 °C in the electrochemical reactor. This means that a substantial amount of dissolved CO2 is available to be converted at the reductive electrode." Furthermore, Goetheer explains, since the dissolved CO2 is reduced to products, a shift in equilibria is brought about. Thus, an elegant method is obtained, where already at lower temperatures the desorption can be effectively used for the transformation of CO2 into value-added chemicals. "Moreover, the temperatures we are looking for might already be there as the result of heating up by the ohmic losses in the electrochemical cell. Or they can be reached by using waste heat. At the TNO labs in Delft, the VoltaChem program is aimed at further optimization of the hydrodynamics within the electrolyzer, to make the most use of this methodology."
 The first series of experiments on the synthesis of formic acid as a platform chemical have demonstrated the viability of the integration concept. But, primarily depending on the potential difference and the type of electrocatalyst, many other conversion options are available. Goetheer mentions carbon monoxide (CO) and ethylene (C2H4) as prime examples of feedstock for the synthesis of bulk chemicals, such as fuels and polymers.
The first series of experiments on the synthesis of formic acid as a platform chemical have demonstrated the viability of the integration concept. But, primarily depending on the potential difference and the type of electrocatalyst, many other conversion options are available. Goetheer mentions carbon monoxide (CO) and ethylene (C2H4) as prime examples of feedstock for the synthesis of bulk chemicals, such as fuels and polymers.
Towards 80% of Faradaic efficiency
In the conversion towards formic acid, Goetheer and his team have already demonstrated impressive conversion figures of more than 50% in terms of Faradaic efficiency - meaning that more than half of the available electrons are actually used for the desired CO2 conversion. It is not realistic to assume that 100% of Faradaic efficiency can be reached, due to the formation of unwanted by-products. But Goetheer is confident that the performance of 80% is within reach. In combination with improving the current density, this will enable sufficiently high conversion rates. "There's quite some work ahead of us, for instance in improving the electrode and reactor design, and in-process optimization with regard to electron transfer, mass transport, and hydrodynamics. But I'm confident we will get there." He adds that fundamental improvements are not needed to bring the concept to higher TRL levels. "Based on the existing knowledge of electrochemical materials and processes, we see very good opportunities to bring this concept to maturity."
Start New Shared Research Program
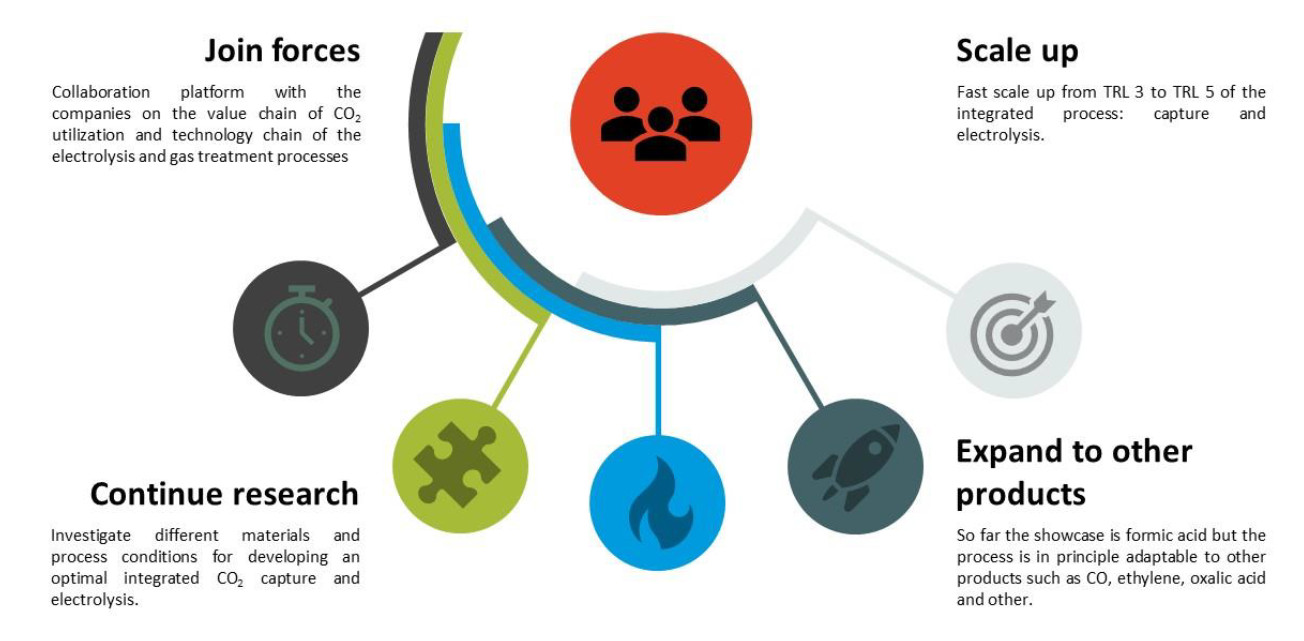 The plan now is to initiate a shared research program in CO2 capture/conversion integration where VoltaChem will boost the development together with the chemical industry and suppliers of electrochemical equipment. The program will have a strong international dimension since not only are the main players in the chemical industry large multinational companies, important builders of electrolysis equipment are also located abroad.
The plan now is to initiate a shared research program in CO2 capture/conversion integration where VoltaChem will boost the development together with the chemical industry and suppliers of electrochemical equipment. The program will have a strong international dimension since not only are the main players in the chemical industry large multinational companies, important builders of electrolysis equipment are also located abroad.
The program thus will offer companies access to a relevant network of knowledge and expertise.
Want to join our discussion?
VoltaChem invites companies to discuss the opportunities of the proposed shared research program during a meeting to be held on November 29th at TNO, Leeghwaterstraat 44 in Delft. Please let us know if you are interested or are not able to attend but would like to keep in touch.
Contact Willem Frens our Business Development Manager of the Power-to-Chemicals program
Share this page: